2.3 Effect of Furnace Atmosphere There was no significant difference in the experimental results when the slag alkalinity of the samples prepared using the sintered magnesia as raw material was 4.
3 Conclusions When the carbon content is in the range of 10% to 20%, the denser layer of MgO formed will increase as the carbon content decreases.
The oxygen that oxidizes Mg(g) to form a dense layer of MgO is provided by molten slag through the molten metal. Therefore, all factors that affect the oxygen activity in molten slag and molten metal will affect the formation of dense MgO layers.
The atmosphere in the furnace has no significant effect on the formation of the Mg dense layer.
Compared with fused magnesia, when sintered magnesia is used as raw material, MgO dense layer is more easily formed on the surface of the refractory material.
Sodium Tert-Butoxide Chemical Information
Density 1,104 g/cm3
Boiling Point 180°C/1mmHg
Melting Point 180 °C
Molecular Formula C4H9NaO
Molecular Weight 96.103
Flash Point 12°C
Storage condition Flammables area
Water Solubility reacts
Sodium tert-butoxide Structure
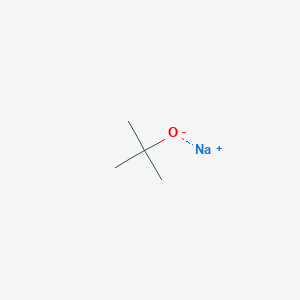
Sodium Tert-butoxide Application
1. Used as an intermediate for organic synthesis and Pharmaceutical Intermediates
2. As a strong base, it is widely used in the condensation, rearrangement and ring opening reactions in organic synthesis such as chemical, pharmaceutical and pesticide.
Sodium Tert-butoxide CAS No.865-48-5
Sodium Tert-Butoxide,Sodium Tert Butoxide Cas No,Sodium Tert Butoxide Synthesis,Sodium Tert-Butoxide Mechanism
ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com