This paper reviews the current status and research progress of electro-galvanized nickel-nickel alloys for automotive parts, summarizes the composition of acidic plating baths and alkaline plating solutions in major international electroplating additive plants, and briefly analyzes the high protective properties of zinc-nickel alloy electroplating. The process was reviewed and the development direction of zinc-nickel alloy plating for automotive parts was proposed.
Introduction
The development of science and technology and modern industry has become more and more demanding for protective coatings, and the traditional electroplated zinc layer can not fully meet the requirements. In recent years, the research and application of zinc alloys has become more and more extensive. Zinc-nickel alloys have been widely used in the automotive industry, aircraft manufacturing and military products due to their good resistance to medium and high temperature and excellent corrosion resistance. Electroplated automotive steel plates and automotive fasteners are used in electroplated zinc-nickel alloys abroad. Zinc-nickel alloy plating was first successful in Japan and Germany. There are more and more parts that are currently protected by zinc-nickel alloys in the automotive industry. After electroplating of zinc-nickel alloy, even if the coating is very thin, it has high corrosion resistance.
1 Zinc-nickel alloy plating advantages
Electroplated zinc has been widely used as a protective coating in automotive parts. Table 1 compares the performance of zinc plating and zinc-nickel alloy plating.
The main advantages of the electroplated zinc-niobium-nickel-nickel alloy plating process are:
1 The protective sorghum is more than twice as high as the zinc coating. It is passivated by white or black and the corrosion resistance is greatly improved.
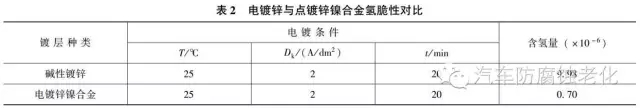
2 Electroplating process has little hydrogen embrittlement and almost no hydrogen embrittlement. It is suitable for electroplating of high-strength steel. Table 2 compares the hydrogen content in electro-galvanized and electro-galvanized nickel alloy coatings.
3 The mechanical properties are good. After electroplating zinc-nickel alloy, the yield strength, tensile strength and elongation of the steel are not changed, and the plasticity is good.
4 Good adhesion to the substrate, the coating has good solderability.
5 The plating solution is simple in composition, easy to use and easy to maintain.
6 The plating solution is low in toxicity and is conducive to environmental protection.
7 The coating has high hardness and scratch resistance.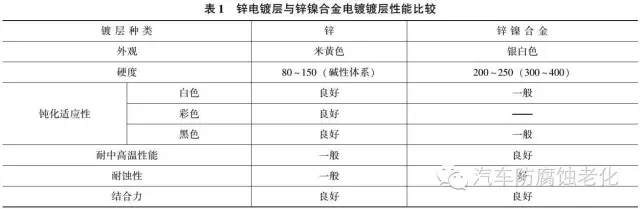
2 High protective analysis of zinc-nickel alloy
Table 3 is a comparison of the corrosion resistance of the zinc plating layer and the zinc-nickel alloy plating layer.
It can be seen from Table 3 that zinc-nickel alloy has stronger protective performance. The main reasons for zinc-nickel alloy as excellent protective coating are:
1 The stable potential is lower than that of iron, and it has electrochemical protection for the steel substrate as an anodic coating.
2 The coating is finely crystallized, which has partial chemical protection to the steel matrix and delays the corrosion rate.
3 It can be seen from Fig. 1 that due to the presence of a small amount of nickel, the crystal structure of the plating layer is preferentially oriented, which effectively hinders the corrosion reaction and improves the corrosion resistance.
4 After passivation treatment, the potential in the alloy is more concentrated than the positive metal nickel to form an anti-corrosion barrier layer, and the protection is improved.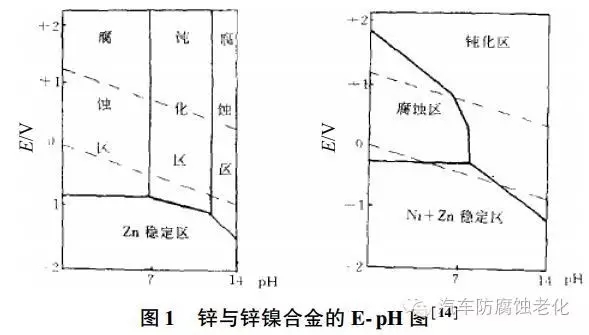
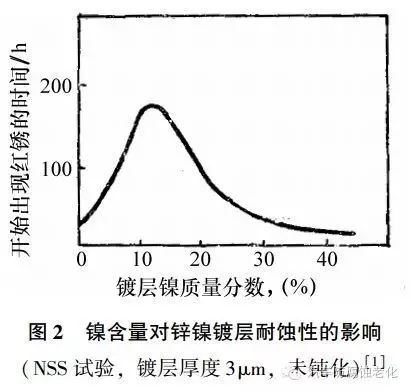
In summary, the presence of nickel improves the coating performance, and Figure 2 shows the effect of nickel content on the corrosion resistance of zinc-nickel coating. It can be seen from the figure that when the nickel content is 13%, the corrosion resistance of zinc-nickel alloy is 6 times that of zinc coating, and the protective performance is better than that of aluminum, cadmium and cadmium titanium alloy, which can be used for highly protective parts or as cadmium. The coating contains an alloy containing less than 15% nickel. The coating is an anodic protective layer for the steel substrate, and the corrosion process is controlled by the anode. The zinc deficiency on the surface improves the barrier layer and reduces the dissolution rate of the anode. The coating has good corrosion resistance and is easy to be chrome-plated or passivated. The zinc-nickel coating with a nickel content higher than 15% is a cathodic protective layer relative to the steel substrate, the chrome plating is difficult, the passivation solution is difficult, and the nickel content is too high and the corrosion resistance is decreased.
3 zinc-nickel alloy plating process
The co-deposition of zinc-nickel alloy belongs to abnormal co-deposition. The zinc-nickel alloy electroplating process is mainly divided into four parts: pre-treatment, electroplating, passivation treatment and dehydrogenation treatment.
3.1 pre-processing
The pre-treatment quality directly determines the protective performance of the coating. The zinc-nickel alloy pre-treatment process is basically similar to the electro-galvanizing process. The general pre-treatment process for electroplating zinc-nickel alloy is:
Pre-plating inspection→mounting→rust removal→water washing→water washing→chemical degreasing or electrochemical degreasing→hot water washing (70~80°C)→water washing→strong corrosion→water washing→water washing→weak etching→water washing→water washing→electroplating .3.2 electroplated zinc-nickel alloy
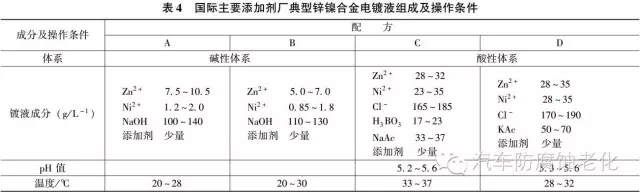
Table 4 shows the composition and operating conditions of typical zinc-nickel alloy plating solutions for some major international additive manufacturers.
Alkaline zinc-nickel alloy plating has the characteristics that acid zinc-nickel cannot match:
1 The composition is uniform over a wide range of current densities and has excellent dispersing ability.
2 Alkaline plating bath has a certain protective effect on the unplated part of the tubular part, while the acidic plating solution accelerates the corrosion of those unplated surfaces.
3 Low cost and easy disposal of waste liquid.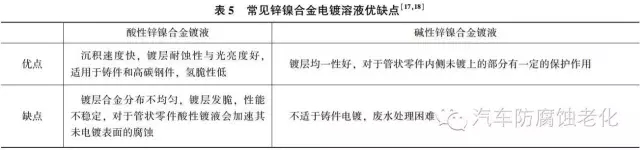
3.3 passivation treatment
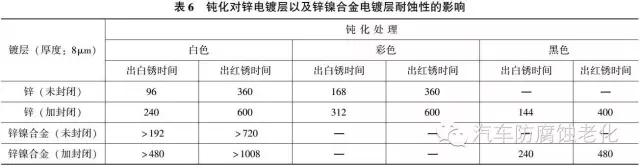
The passivation treatment of zinc-nickel alloy is mainly black and white, and the passivation film treated by chromate passivation has a corrosion resistance higher than that of the passivation film of the galvanized layer. However, it is more difficult to form a passivation film on a zinc-nickel alloy than a galvanized layer, and it becomes more difficult as the nickel content in the alloy increases. The nickel content is within 10%, and the passivation is relatively easy, and the nickel content is about 13%. Passivation is difficult, and the nickel content exceeds 16%, which is difficult to passivate.
3.4 dehydrogenation treatment
The zinc-nickel alloy coating has a lower hydrogen embrittlement than the galvanized layer (Table 2), but when the zinc-nickel alloy is used for military products, high-strength steel or spring parts, dehydrogenation is required.
4 zinc-nickel alloy deplating
Unqualified electrogalvanized nickel parts need to be deplated in order to reduce the cost of secondary use. Choosing a suitable stripping solution can result in no significant changes in material properties. The zinc-nickel alloy stripping solution [11,18] of the general steel parts has a volume fraction of 10% hydrochloric acid and the working temperature is 15~35 °C; the zinc-nickel alloy stripping solution on the aluminum part is 500g/L of nitric acid, the working temperature It is room temperature; the deplating solution of steel plated parts and elastic parts with tensile strength greater than or equal to 1050 MPa is 100-200 g/L sodium nitrite and 200-300 g/L sodium hydroxide, and the working temperature is 100-150 °C.
5 Outlook
Zinc-nickel alloy coatings have become more and more widely used in automotive parts. Due to their good protection and cost performance, excellent corrosion resistance and low hydrogen embrittlement, they have become excellent cadmium plating. The wide application of zinc-nickel alloys is of great significance for improving the quality of protective layers, saving metal resources, reducing pollution and reducing costs for automotive parts. The future development direction is:
(1) Development of color zinc-nickel alloy plating
At present, the appearance of zinc-nickel alloy coatings in the market mainly includes white and black. With the advancement of technology, not only the more demanding requirements for protection are put forward, but also the appearance requirements are more demanding. The development of zinc-nickel coatings of various colors is potential. It must be done.
(2) Acid zinc-nickel alloy system
At present, the main international and domestic electroplating additive plants mainly adopt alkaline zinc-nickel alloy system. The acid zinc-nickel alloy system is less used due to unstable plating solution, etc., with the corrosion resistance requirements of the automobile main engine factory for castings and high-strength steel. The improvement of the acid zinc-nickel alloy system will have a large market space.
(3) Corrosion resistance of black zinc-nickel alloy needs to be improved
Due to the black appearance of zinc and passivation agent when the surface of black zinc-nickel alloy coating is passivated, the corrosion resistance of black zinc-nickel alloy coating used on the market is much lower than that of white zinc-nickel alloy. The corrosion resistance of black zinc-nickel alloy is to be treated. Further improve.
60%65% Dially Dimethyl Ammonium Chloride DADMAC And DMDAAC CAS 7398-69-8
Water-Treatment Chemical,Dadmac Polymer For Polyquaternium-6,Dially Dimethyl Ammonium Chloride 65%,Easy Dissolve Poly Dadmac
ZHEJIANG XINHAITIAN BIO-TECHNOLOGY CO.,LTD. , https://www.dadmacxht.com