Just renovated the house, did not live long, wall peeling, chalking, shedding, will give you a lot of trouble, will blame the paint is not good, or the workers are sloppy. However, you may not know that the fundamental reason for wall peeling, pulverization, and shedding is not the level of paint and construction, but the result of anti-alkali treatment that you did during the renovation. Following Xiaobian see how to prevent the wall back to alkali? How to do the wall back to alkali ?

How to prevent wall back alkali
When painting the wall, sometimes we will encounter some minor troubles, such as the upper wall of the paint absorbed too quickly, the paint component can not brush the predetermined area; soon after the brush appeared discoloration problems, and even the surface layer off , Blocks, mildew, often let us helpless. Originally, no matter the new wall or the old wall in the living room, due to excessive alkalization due to excessive water content, it is even more so in the wet environment, which is the fundamental reason that affects the quality of the paint. Applying a layer of water-based sealing primer before brushing the top coat can not only save the decoration cost, but also has the advantage of being more effective when used in the long-term and decorative effect. The excellent sealing properties of these primers make them effective against the penetration of basic substances in the wall, preventing the precipitation of water-soluble salts and preventing the return of alkali to the walls. At the same time, the good adhesion of the primer makes the wall more smooth, making the topcoat easier to apply and reducing the amount of topcoat used. In this way, the wall surface not only avoids the paint disease, but also helps to maintain the brushing effect for a longer time.
It can be seen that brushing the primer has a crucial influence on the quality of the wall surface. The owners of the newly renovated owners have to do a better job in the treatment of the basement of the wall due to the lack of knowledge and experience in the decoration. Unnecessary trouble caused rework troubles.
How to do the wall back to alkali?
1. Prevent the production of water-soluble ions
The solution is to convert calcium hydroxide to calcium carbonate.
(1) Solution mechanism: The main ion source for returning alkali is a large amount of OH- in the lime slurry. After being converted into calcium carbonate, since calcium carbonate is insoluble in water, it will not produce a large amount of ions that leak out with water, ie, it will not generate Alkali.
(2) Problems to be solved: As the calcium carbonate and the calcium hydroxide are dried, their crystal structure, structure and mechanical properties are different, which may cause the calcium carbonate filling slurry to affect the adhesiveness of the wall. It can be adjusted by adding an appropriate amount of viscous additives, such as rice paste or latex, which is similar to ancient ones.

2. Prevent ions from being carried out with moisture
1). Calcium bicarbonate solution instead of calcium hydroxide solution for drainage Drainage with calcium bicarbonate solution can introduce bicarbonate ions. The hydroxide ions in the next calcium hydroxide solution will react with bicarbonate ions to form carbonate ions and combine with calcium ions to form calcium carbonate precipitates. This calcium carbonate precipitate will form a protective layer on the inner surface of bricks, reducing The amount of ions that the moisture brings to the outer surface of the wall during the drying process, and has a similar effect to the calcium carbonate layer in 3.2.2.
2). Add calcium carbonate between the wall tiles and calcium hydroxide, the second does not return to alkali
(1) The reason for the secondary return to alkali: During the process of water infiltrating ions, it will continuously evaporate, which will cause the part of the ions originally dissolved in water to react with carbon dioxide, and will continuously precipitate out to produce alkali. After the construction is completed, if the water is used, it has been washed. The returned alkali is generated. The water dissolves and removes the salt precipitated on the surface. It also penetrates into the brick and brick joints, dissolves the calcium hydroxide again, and repeats the initial alkali return process during the drying process, resulting in secondary alkali return.
(2) Solve the mechanism: Add the calcium carbonate layer on the inner surface of the brick by perfusion method first, and then use the original construction method to infuse calcium hydroxide. On the one hand, in the flushing process after returning alkali, by controlling the depth of penetration of the rinsing agent into the wall, the moisture only contacts the calcium carbonate layer and avoids contact with the calcium hydroxide layer, thereby preventing the moisture from bringing the ions out; on the other hand, This method also ensures that the wall filling material is mainly calcium hydroxide and will not affect its mechanical effect.
3. The emitted ions do not appear white after evaporation of water on the surface
The following analysis was performed based on the fact that the crystalline salt block was colorless and easy to drop.
(1) Back to alkali properties: The particles precipitated by evaporation of water are very small, so they are white powder and adhere to the surface of the wall, impeding the appearance and being difficult to remove.

(2) Solution mechanism: The addition of appropriate components allows the salt (or alkali) to precipitate as granular crystals with large particles and transparent (like granulated salt), which will also facilitate physical removal.
(3) Problems to be solved: Due to various problems concerning the amount, properties and performance of additives, the selection of such effective additives is still difficult.
4. Wash with weak acid to alkali white
(1) Solution mechanism: The white powder formed on the surface of the brick body can be dissolved and form a neutral salt under the action of weak acid or dilute acid. This salt can be reduced along with the solution along the surface of the brick wall in the cleaning process to reduce the wall thickness. The remnants of the surface, at the same time, the dissolution process is the recrystallization of salt on the wall surface. And because the neutral salt has better crystallinity with respect to the alkali or basic salt, the crystal is more transparent, and the recrystallization process will make the neutral salt crystal grain larger. So this process will make the white surface of the wall relieve or disappear.
(2) Embodiment: Spraying the surface of the wall with dilute acetic acid or hydrochloric acid solution should try to make the moisture immersed into the shallow depth of the brick body, so as to avoid the secondary exudation of the internal ions.
(3) Auxiliary measures: In combination with the method of adding a calcium carbonate layer between the wall brick and the calcium hydroxide, the small amount of moisture that penetrates into the wall during the washing can not be brought out again when the water evaporates, and the secondary returning alkali is avoided.

Editing summary: The above is related to how the wall back to alkali, how to do , because everyone needs are different, so the choice is different, the above information for your reference. Hope to help friends who have this need!
Small apartment wall decoration wall decoration DIY wall storage European style wall decoration
Calcium Ethoxide Basic Information
CAS: 2914-17-2
MF: C4H10CaO2
MW: 130.2
EINECS: 220-828-8
Mol File: 2914-17-2.mol
Calcium Ethoxide Chemical Properties
Melting point >170°C (dec.)
Safety Information
Risk Statements 11-14-36
Safety Statements 8-16-26-43
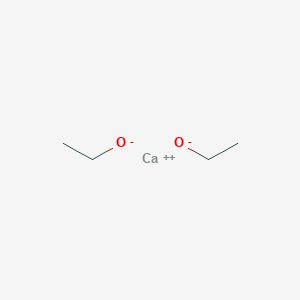
Calcium Ethoxide CAS No.2914-17-2
calcium ethanoate,calcium ethanoate and ethanol,calcium ethanoate formula,calcium ethanoate and ethanol reaction,calcium ethanoate balanced equation,calcium ethanoate bonding
ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com